CDW-R Data Extraction and Provisioning Services
The CDW-R provides a range of services, from aggregate counts to full data extracts to collaborating with larger groups on the BUMC campus to increase their research data infrastructure.
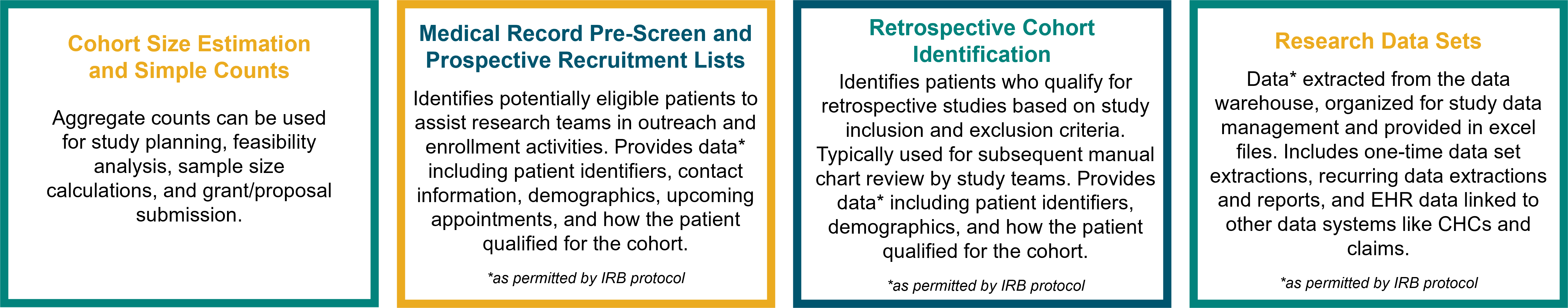
Cohort Size Estimation
CDW-R provides investigators with aggregate, simple count data (e.g., the total number of hospitalized patients with pneumonia in 2024) to include in grant applications or conduct sample size calculations. Simple count data are provided to investigators with the expectation that funding for CDW-R services is included in proposal budgets. Simple count data requests do not require IRB approval. Counts fewer than six cannot be reported due to privacy concerns. Request aggregate, simple counts by submitting a CDW-R simple counts request.
Data Extracts & Data Sets
With appropriate approvals, CDW-R analysts provide extracts of data housed in the Research Data Warehouse for retrospective or prospective research studies.
- Recruitment Support via Medical Record Pre-Screens and Preliminarily Eligible Patient Lists: CDW-R medical record pre-screen and recruitment list services provide study teams with a list of patients in the Epic medical record system who are preliminarily eligible for your study to facilitate enrollment activities – potentially mitigating the need for a manual chart review. Recruitment lists supplied to study teams generally include demographics, how patients qualified for the cohort, and contact information for study team outreach.
- Retrospective Cohort Identification: For retrospective chart review studies where manual chart review in Epic is required, CDW-R will identify the study cohort according to your study inclusion and exclusion criteria and provide the study team with a list of patients for subsequent manual chart review – increasing efficiency, reducing manual work, and improving consistent cohort capture.
- Research Data Sets: CDW-R provides a range of research data set extraction services suitable for research analysis, including provisioning de-identified data sets, HIPAA limited data sets, and identifiable data sets. For example, a research team studying acute health care utilization among patients with neck abscesses may request a data set that contains patient demographics; emergency department visit dates; in-patient admission and discharge dates; operating room information; and lab values such as the value and date of the most recent white blood cell count.
Visit How to Request Data for the CDW-R research data extraction request process and requirements.
Research Data Request Development Consultations
CDW-R provides research data extraction request development support to identify available data elements to answer research questions; assist with ensuring data needs are accurately reflected on your BMC/BUMC INSPIR application; determine if a subsequent manual chart review would be required for your study; and prepare to submit a formal CDW-R research data extraction request.
All investigators, particularly new investigators or those with early experience working with EHR data, are encouraged to contact CDW-R in advance of IRB submission to confirm available data and discuss feasibility, scope, cost estimates, and how to optimize the data request. Reach out to cdw@bmc.org with questions.
- For efficient service, please provide the following when contacting CDW-R: The BMC/BUMC IRB H- Number, a summary of the services/data you anticipate needing from the CDW-R team, and your specific questions.
- For inquiries that cannot be address by email, CDW-R offers one (1) initial consultation at no charge for every study. This consultation can take place prior to your study's IRB approval - and can be helpful to determine what data should be included in your IRB submission. Subsequent consultations and request development support services are billed at our hourly fees.
Additional Data Services
While creating these data outputs and deliverables for study teams, the CDW-R also facilitates:
- Applying study cohort inclusion and exclusion criteria.
- Assigning study IDs.
- Basic cohort matching.
- Creating and managing patient keys and master codes, with or without providing access to the study team.
- Providing subsequent data extracts for the same cohort (whether study team has a master code or not).
- Developing recurring and automated data pulls.
- Leveraging CDW-R's clinically-informed algorithms and phenotypes for identifying patients, characteristics, and conditions.
- Merging study team's external data or primary data collection files with Research Data Warehouse extracts.
- With appropriate approvals, linking BMC EPIC clinical data with CHC OCHIN data for patients with health care encounters at BMC and participating Boston HealthNet CHCs.
